2011-3-17 2:24:19
views
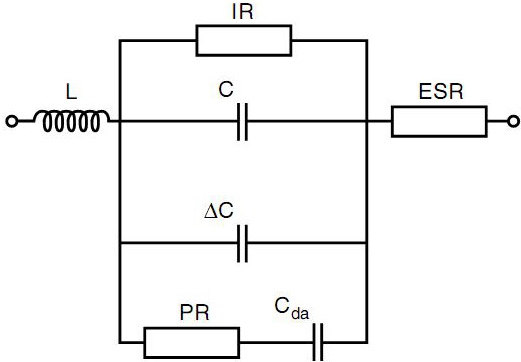
C = nominal value of the capacitor
L = inductance (leads, metallization, winding)
ESR = equivalent series resistance (leads, metallization, metal spraying)
IR = insulation resistance (properties of the dielectric material)
∆C = capacitance change (depending on changes in temperature, DC voltage and/or frequency)
PR = dielectric polarization resistance
Cda = dielectric absorption
2011-3-16 2:26:9
views
Capacitors loaded with pulses with fast rise or fall times (high dU/dt) will be exposed to high current pulses. In order not to overload the internal connections the current must be limited. The current limits for a specifc type are dependent upon:
- Amplitude and form of the pulse
- Rated voltage of the capacitor
- Capacitance
- Geometrical configuration of the winding
dU/dt = UR/(R x C)
UR = Rated voltage
R = Discharge resistor
C = Rated capacitance
2011-3-15 2:27:44
views
The reliability of a capacitor is mainly a function of:
- The construction; dielectric material and its thickness
- The manufacturing process
- The application; electrical stress and temperature
2011-3-12 12:8:34
views
Jb Capacitor Manufactures X2 Metallized Polypropylene Film Capacitor ,JFV-X2 Metallized Polypropylene Film Capacitor is jb’s new products
Applications :
- Ideal for use in line bypass, antenna coupling, across-the-line and spark killer circuits
- Available for EMI filter
- Switching power supply applications.
Features:
- Non-inductive,(UL940V-0) plastic case sealed with epoxy resin,
- Provides interference suppression with CE & TUV and UL approvals.
- High dv/dt capabilities.
2011-3-11 12:1:55
views
A colour code was used on polyester capacitors for many years. It is now obsolete, but of course there are many still around. The colours should be read like the resistor code, the top three colour bands giving the value in pF. Ignore the 4th band (tolerance) and 5th band (voltage rating).
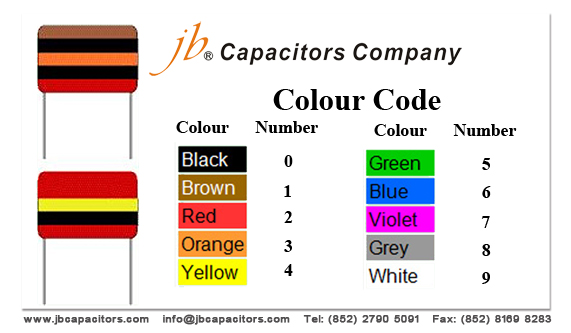
For example:brown, black, orange means 10000pF = 10nF = 0.01µF.
Note that there are no gaps between the colour bands, so 2 identical bands actually appear as a wide band.
For example:wide red, yellow means 220nF = 0.22µF.
2011-3-10 11:55:49
views
Capacitors in a parallel configuration each have the same applied voltage. Their capacitances add up. Charge is apportioned among them by size. Using the schematic diagram to visualize parallel plates, it is apparent that each capacitor contributes to the total surface area.
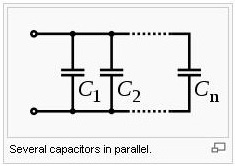
Ceq=C1+C2+……+Cn
2011-3-9 11:43:5
views
The following rules should be observed when handling aluminum electrolytic capacitors:
Any escaping electrolyte should not come into contact with eyes or skin.
If electrolyte does come into contact with the skin, wash the affected parts immediately with running water. If the eyes are affected, rinse them for 10 minutes with plenty of water. If symptoms persist, seek medical treatment.
...
2011-3-8 15:36:54
views
Basically the SMD series have the same electrical characteristics as the analogous through-hole WIMA capacitors. Compared to ceramic or tantalum dielectrics WIMA SMD capacitors have a number of other outstanding qualities:
- favourable pulse rise time
- low ESR
- low dielectric absorption
- available in high voltage series
- large capacitance spectrum
- stand up to high mechanical stress
- good long-term stability
2011-3-7 15:28:40
views
jb capacitors company produce X2 metallized polypropylene film capacitors--JFV series, with high quality and very short lead time & aggressive price.
Focus on overseas customers, JFV series X2 metallized polypropylene film capacitors is one of our best sellers, with safety approvals, widely used in powersupply, power meters, white goods, ballast, lighting products, etc..
JFV--X2 Metallized polypropylene film capacitors,
cross Epcos B81130, Vishay MKP3382, Evox Rifa PHE840M, Phikor PCX2 337, Wima MP3 X2, Panasonic ECQU, Arcotronics(Kemet) R46, jb capacitors JFO series

Datasheet: http://www.jbcapacitors.com/pdf/JFV-X2-Metallized-Polypropylene-Film-Capacitor.pdf
2011-3-2 10:41:58
views
The capacitors are usable for radial manual insertion on PCB. The fixation on the board by double kinked leads prevents that the component jumps out of the PCB during transport.
The components with lead diameter of 0.6 mm are usable for being inserted in punched holes with nominal diameter of 0.8 mm and the components with lead diameter of 0.8 mm are usable for being inserted in punched holes with nominal diameter of 1.0 mm.
The pitch is specified on the top of the leads. After manufacturing, the products meet the specification. Although special care is taken to the packaging, deviations may occur due to transport.




