Introduction to Aluminum Electrolytic Capacitors - Part 4
[2]-2 Forming (Anode Oxidation)
The "Forming" process is defined by creating an electrically insulating oxide (to provide the withstand voltage) on the aluminum surface by performing anode oxidation in the electrolytic solution used for the growth. The produced chemical film is used as the anode thin film.
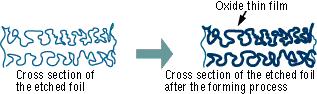
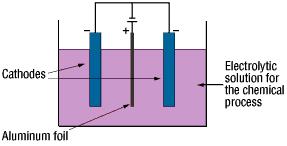
The anode oxidation, as follow shown, is produced by applying a voltage to the submerged foil found in the electrolytic solution used for growing the oxide film. Generally, the electrolytic solution is an aqueous solution such as ammonium boric acid, ammonium phosphate, or ammonium adipic for acid.
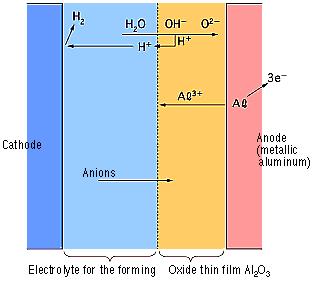
During the anode oxidation (DC electrolysis), AL2O3 is produced by a reaction between the water and the aluminum's Al3+ ions. The thickness of the grown thin film is nearly proportional to the applied voltage] with approximately 1.0 to 1.4 nm per volt. The chemical reactions on the anode side and the cathode side are as follows.
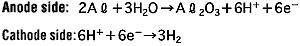
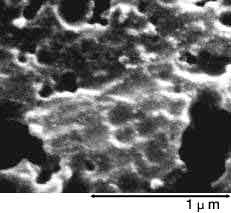
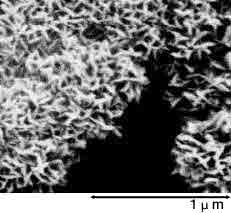
Below photographs show a magnified view of the oxide layer produced through anode oxidation.
| Low-voltage forming foil | High-voltage forming foil |
|
|
|
| Photograph of surface | Photograph of surface |






0 Comment so far
Leave a reply