2018-2-7 17:54:33
views
With the Chinese New Year coming, jb launched four new series of audio capacitors.
They are all good quality e-caps, can bring your audio system a great performance.
Now click below series name to check their PDF datasheets!
Hope you will bring us many of new orders in Dog of the year. Happy CNY !
| Series | JAC | JAD | JAE | JAF |
| Items | Performance Characteristics |
| Operating Temperature Range | -40℃~+105℃ |
| Load Life | 1000 hours |
| Capacitance Tolerance | ±10% |
| Leakage Current (μA) | MAX. 0.03CV + 3μA After 5 minutes application of rated working voltage |
| MAX Dissipation Factor | MAX 4% at 1KHz | MAX 5% at 1KHz | MAX 10% at 1KHz | MAX 4% at 120Hz |
| Features | Bi-Polarized |
| Specially produced for Cross-Over Networks with high fidelity audio system |
| High-quality crossover non-polar aluminum electrolytic capacitors |
| Application | Audio Converters and Dividers (partials), Audio amp, Automotive Electronics Products, speaker |

2018-1-16 0:7:3
views
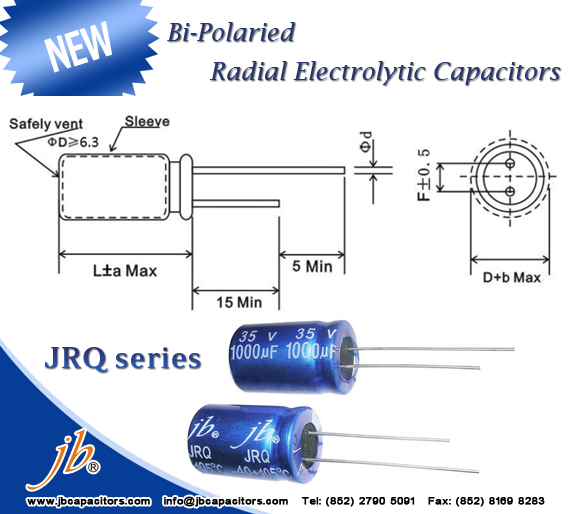
Good news! Recently we released another Radial electrolytic capacitors---JRQ series. The support is very good.
Are you looking for such Bi-Polaried Radial E-cap? Welcome to send us inquiry at once.
JRQ - 1000H at 105C, Bi-Polaried Radial Aluminum Electrolytic Capacitor
* Bi-Polarized
* 105C 1000 hours
* Used in polarity reverse and change circuits
* Operating Temperature Range: -40C~+105C
* Capacitance Tolerance: (25°C, 120Hz) ±20%
* Leakage Current: (μA) I≤0.03CV + 3(μA) (1minute)
PDF datasheet: http://www.jbcapacitors.com/pdf/JRQ-105-Bi-Polaried-Radial-Aluminum-Electrolytic-Capacitors.pdf
From jb Capacitors Company
-------We're just your best choice
2017-11-23 0:14:48
views
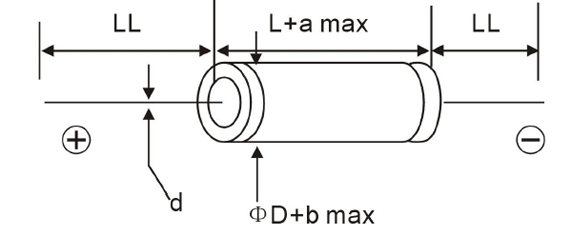
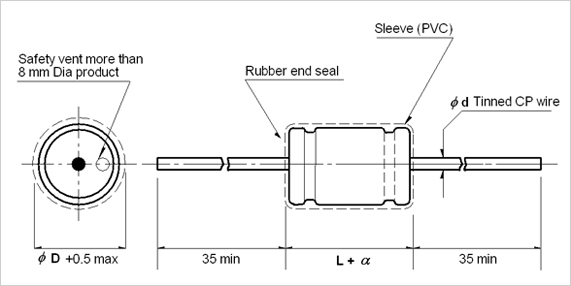
To meet market needs, we released newest Axial lead E-cap--JAA and JAB series, both series are 2000H at 85C. Please find detail difference as below:
JAA - 2000H at 85°C, Axial Aluminum Electrolytic Capacitors (Low Leakage)
• Load life of 2000 hours at 85°C
• Low Leakage Current
• 2 Rubber Types
• For general purpose application
• RoHS Directive Compliant
PDF datasheet: http://www.jbcapacitors.com/pdf/JAA-85-Axial-Aluminum-Electrolytic-Capacitors-Low-Leakage.pdf
JAB - 2000H at 85°C, Axial Aluminum Electrolytic Capacitors
• Load life of 2000 hours at 85°C
• Low Voltage
• 2 Rubber Types
• For general purpose application
• RoHS Directive Compliant
PDF datasheet: http://www.jbcapacitors.com/pdf/JAB-85-Axial-Aluminum-Electrolytic-Capacitors.pdf
JAA, JAB series Drawing:

From jb Capacitors Company
-------We're just your best choice
2017-9-28 1:16:48
views
Elektrolitik kondansatorlerde dielektrik madde olarak asit borik eriyigi ya da borakslı elektrolitler bulunur. İletken levhalar ise aluminyum ya da tantalyum plakalardır. Elektrolitik kondansatorler, kutuplu ya da kutupsuz olarak uretilirler. Kutuplu olan kondansatorler DC devrelere baglanırken artı ve eksi uclara dikkat edilmelidir. Yanlıs yapılan baglantılarda anotta bulunan oksit tabakası metal yuzeyi kısa devre edip, yuksek ısı olusumuna ve kondansatorun patlamasına yol acabilir.
Elektrolitik kondansatorler sıvılı tip ve kuru tip olarak ikiye ayrılır. Sıvılı tip elektrolitik kondansatorler sadece DC devrelerde kullanılabilen, pozitif levha olarak aluminyum barındıran kondansatorlerdir.
Bu tip kondansatorlere DC akım uygulandıgında, pozitif levha üzerinde yalıtkan bir oksit tabakası oluşarak dielektrik gibi davranır. Olusan bu tabaka cok ince oldugundan kondansatorun kapasitesi yuksek olur. Kuru tip elektrolitik kondansatorlerde ise elektrolitik sıvı yerine boraks eriyigi emdirilmis kagıt ya da bez kullanılır.
Elektrolitik kondansatorlerde bulunan elektrolitik sıvısı asırı sıcak nedeniyle zamanla kurumaya basladıgından, kondansatorun kapasite degeri dusebilir ve bu durum hassas devrelerin calısma sisteminde arızalara neden olabilir.

2017-9-20 23:48:28
views
Since 2005, jb Capacitors Company has been specialized in production of Aluminum Electrolytic Capacitors, today we would like to introduce you JCS - the hot seller of SMD Aluminum Electrolytic Capacitors.
SMD E-Cap looks tiny and special, they can be widely used in so many ways, they’re much easier to place using automated assembly equipment.
JCS is round type, it has the greatest quality and favorable price, also short lead time. You can see the specification form down below.
Here is JCS PDF datasheet, you might want to check it out! http://www.jbcapacitors.com/pdf/JCS-2000H-at-85-SMD-Aluminum-Electrolytic-Capacitor.pdf
| | JCS |
| Operating Temperature | -40°C~ +85°C |
| Voltage Range | 4V ~ 100V.DC |
| Capacitance Range | 0.1 ~ 10000μF |
| Capacitance Tolerance | ±20% at 120Hz, 20°C |
| Leakage Current | Leakage current (Φ4~Φ10) ≤0.01CV or 3μA, whichever is greater.(after 2 minutes application of rated voltage) |
| Leakage current (Φ12.5~Φ16) ≤0.03CV or 4μA, whichever is greater.(after 1 minutes application of rated voltage) |

2017-8-24 1:43:7
views
I bring you another our strong series radial type aluminum electrolytic capacitors JRK serires, it is miniature, height 7mm THT type aluminum electrolytic capacitors.
We have very competitive prices and short lead time(around 3~4 weeks), Check below our competitive offer, welcome your RFQs.
JRK-2000hours at 105C', Radial aluminum electrolytic capacitors, miniature size, height 7mm
100uF 6.3V +/-20% DxL=4*7mm Bulk RoHS QTY: 50kpcs~99kpcs U$5.2/Kpcs QTY:100kpcs or more US$4.6/kpcs
100uF 10V +/-20% DxL=5*7mm Bulk RoHS QTY: 50kpcs~99kpcs U$6.0/Kpcs QTY:100kpcs or more US$5.2kpcs
100uF 16V +/-20% DxL=5*7mm Bulk RoHS QTY: 50kpcs~99kpcs U$6.3/Kpcs QTY:100kpcs or more US$5.4/kpcs
220uF 10V +/-20% DxL=6.3*7mm Bulk RoHS QTY: 50kpcs~99kpcs US$9.0/kpcs QTY:100kpcs or more US$7.9/kpcs

2017-6-21 18:54:48
views
Do you use or buy Snap-in Aluminum Elco Cap?
We have below two best series, with excellent quality, low MOQ support, Quick lead time, also widely used in Power supplier, Power meter, lighting, amplifier....applications.
JNC - 2000H at 85°C, Snap-in Aluminum Electrolytic Capacitor, for Speaker Network
Can replacement to: Epcso B41231, Nippon Chemi-con SMQ, Nichicon LS, Panasonic TS-UQ, Samwha HC series, Kendeil K26 sereis, Yageo LH series.
JNE - 2000H at 105°C, Miniaturized, Snap-in Aluminum Electrolytic Capacitor
Can replacement to: Epcos B41252, B43515, B43525 Chemi-con KMT/KMR, Nichicon GL, AK, AQ, Panasonic HE series, Kendeil K25 series, Yagoe LG series.
Do you want to try us? Welcome your inquiry or ask for samples, we'll not let you down.
jb Capacitors Company
----We are just your best choice

2017-4-27 1:18:45
views
Please check our below Hot seller series of Radial Aluminum Electrolytic Capacitors.
We will offer most competitive prices, high quality, attractive delivery time, and best customer service.
If you have any inquiry, please send us RFQs asap, let's help you cost down.
| Radial Aluminum Electrolytic Capacitors |
| Series | Load life | Temperature | Remark |
| JRA | 2000H at 85°C, | 85°C | standard type |
| JRB | 2000H at 105°C | 105°C | standard type |
| JRC | 2000H at 105°C | 105°C | Low Impedance |
| JRD | 5000H at 105°C | 105°C | Low Impedance |
| JRG | 10000H at 105°C | 105°C | Exremely Impedance |
We sincerely look forward to your business chance!
jb Capacitors Company
----We are just your best choice

2017-4-19 23:22:48
views
jb Capacitors, a professional manufacturer for aluminum electrolytic capacitors, and film capacitors.
Two factories, 3 branch offices: Taiwan, HongKong, Dongguan.
Our L/T for the aluminum ECap series improved much now. and especially when the quantity is bigger than MOQ, we can be more faster.
Some items we have free samples on stock now, please welcome to ask for free samples for testing!
Add Radial ECap + Large can type ECap picture below.